
By Richard Murphy
The uptake and utilization of selenium depends greatly on the form in which the element is presented and plays a critical role in its bioavailability and efficacy. Selenium supplements are available in two forms: (1) inorganic mineral salts, such as sodium selenite or selenate, and (2) organic forms, such as seleniumenriched yeast.
Selenized yeast, in which selenium is assimilated during a controlled fermentation process, is the most bioavailable organic selenium source. Even though numerous other products are labeled as organic, a consideration of the production methods and basic chemistry behind these claims would indicate otherwise.
Selenium absorption occurs within the small intestine, and while selenoamino acids are absorbed using amino acid and peptide transport mechanisms, the absorption of inorganic selenium such as selenite is less efficient and occurs mainly by passive diffusion. Following absorption, selenium-containing amino acids such as selenomethionine (SeMet) can be incorporated non-specifically into general body proteins in place of methionine and can act as a biological pool for selenium that can be utilized during periods of suboptimal selenium intake.
While SeMet is the predominant selenium species in selenized yeast, recent research has indicated the presence of more than 60 seleniumcontaining species, including 20-plus previously unreported metabolites (Arnaudguilhem et al., 2012).
There is evidence that all of these diverse selenium-containing amino acid intermediaries are recognized as selenium species and utilized intracellularly in the synthesis of selenoproteins. In contrast, inorganic sources that are taken up through the small intestine are either utilized or methylated and subsequently excreted.
The tissue-specific distribution of selenoamino acids can give some indication as to the function in which the accumulated selenium is involved and also to the metabolic fate of the selenium source. Selenocysteine content within tissues is typically associated with selenoenzyme activity as selenocysteine forms the functional core of all selenoenzymes.
The accumulation of SeMet within tissues is an indicator not only of enhanced selenium uptake and retention but also of an endogenous selenium pool that can be utilized during periods of suboptimal selenium intake or during periods of oxidative challenge.
As an added benefit, SeMet incorporated into the tissues of meatproducing animals not only increases the selenium content of the tissue but will also be more available to the consumer of such animal products.
Selenium yeast efficacy
It is a well-accepted fact that even closely related yeast strains have their own unique biochemical and genetic characteristics, and numerous peer reviewed research papers have been published on this topic. One such study examined three commercial preparations of sele- nium enriched yeast and assessed the composition of each product in terms of selenium deposition within the individual yeast fraction.
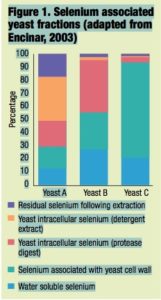 Each product was initially extracted with water and subsequently followed by a variety of enzymatic digestions designed to liberate selenocompounds associated with various polysaccharide and protein portions. These selenocompounds were subsequently separated and speciated by size exclusion chromatography-induc- tively coupled plasma mass spectrome- try and the recoveries in the various fractions from each yeast product were com- pared to each other (Figure 1).
Each product was initially extracted with water and subsequently followed by a variety of enzymatic digestions designed to liberate selenocompounds associated with various polysaccharide and protein portions. These selenocompounds were subsequently separated and speciated by size exclusion chromatography-induc- tively coupled plasma mass spectrome- try and the recoveries in the various fractions from each yeast product were com- pared to each other (Figure 1).
The results monitor the fractionation of the selenocompounds in yeast by using different extraction techniques and illustrate the large variation that exists in the composition of selenium yeast products.
Although there is a very common perception that all selenium yeast preparations are exactly the same, this is not the case. It is clear that the deposition of selenium within yeast is totally different among products. Just as there are differences among yeast strains at a genetic level, there also appear to be fundamental differences in the way yeasts distribute selenium within the cell. It is reason- able to expect, therefore, that these preparations will also differ in parameters such as shelf life, bioavailability and, indeed, toxicology. Rather than viewing these products in exactly the same light, it is clear that they must be seen as distinct selenium preparations.
Selenium metabolism
Regardless of source, selenium requires a metabolic transformation to selenide prior to its assimilation into selenocysteine and subsequent incorporation into selenoproteins. No such intermediate step is necessary for the incorporation of SeMet into general proteins. Consequently, the biological actions of selenium depend not only on the amount but also on the form of the selenium source.
In the case of organic selenium products such as selenized yeast, biological efficacy is more 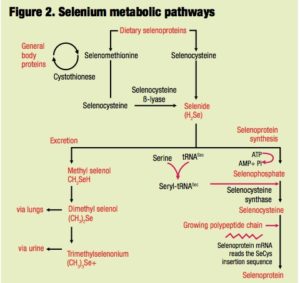 dependent on the accessibility of selenium-containing proteins and peptides present in the preparations. Figure 2 outlines the main metabolic pathways for selenium. It is important to make the distinction between total SeMet and free SeMet because only the free form of this selenoamino acid is available for nonspecific incorporation into proteins. Unless SeMet is liberated as a free amino acid, then regardless of the total selenium and/or total SeMet content, the selenium source must be metabolized to selenide prior to its assimilation into the general selenium pool.
dependent on the accessibility of selenium-containing proteins and peptides present in the preparations. Figure 2 outlines the main metabolic pathways for selenium. It is important to make the distinction between total SeMet and free SeMet because only the free form of this selenoamino acid is available for nonspecific incorporation into proteins. Unless SeMet is liberated as a free amino acid, then regardless of the total selenium and/or total SeMet content, the selenium source must be metabolized to selenide prior to its assimilation into the general selenium pool.
Within the feed industry, there is a mis- conception regarding the total SeMet content of selenium-enriched yeast with the belief that “more is better.” Such arguments have no scientific basis and, indeed, no evidence to indicate the availability of the selenium-containing proteins and peptides within individual products.
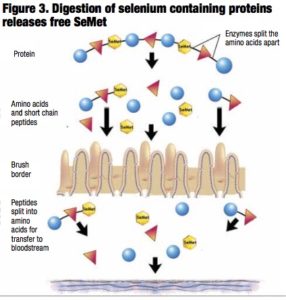 This misconception is based on the belief that SeMet is the “active” component in selenium yeast. While the level of SeMet may vary among products, it is also to be anticipated that the accessibility, digestibility and, thus, the amount of free SeMet that is liberated will also differ (Figure 3).
This misconception is based on the belief that SeMet is the “active” component in selenium yeast. While the level of SeMet may vary among products, it is also to be anticipated that the accessibility, digestibility and, thus, the amount of free SeMet that is liberated will also differ (Figure 3).
Peer-reviewed research has addressed this issue by assessing the digestibility of selenium-containing protein and peptides in selenium-enriched yeast using two-dimensional chromatography and mass spectrometry following in vitro gastrointestinal digestion (Reyes et al., 2006).
Surprisingly, the findings indicated that while approximately 90% of the total selenium was extracted after gastrointestinal digestion, only 34% was quantified in the form of free SeMet amino acid. The rest of the selenium was present in the form of low-, medium- and high-molec- ular weight selenium species that could be detected and further characterized by using two-dimensional chromatography.
Interestingly, most selenium species were in the form of selenium-peptides unspecifically produced by the simulated digestion.
In essence, while the efficiency of gastro- intestinal digestion to break down sele- nopeptides and proteins found in selenium yeast may be high, its efficiency to convert them into free SeMet is much lower. It is important, therefore, for con- sideration be given to the availability of the more than 60 additional selenoamino acids in selenized yeast. These, too, are subject to the same digestive processes that influence the bioavailability of the main selenium species.
While it is difficult to make direct comparisons among selenium yeast products on the basis of their bioavailabilities as a selenium source, we can compare published tissue retention data and use these as an indication of the bioavailability of the individual products.
As part of the European Union product registration process, each selenium yeast manufacturer is required to submit dossiers detailing the efficacy, safety and toxicity of their products. The European Food Safety Authority (EFSA) then assesses the efficacy and safety of the products for all species, as well as the safety for the user, consumer and the environment. The Table shows a summary of the tissue retention data published in the official EFSA opinion on each of the selenium-enriched yeast products authorized for use in the EU.
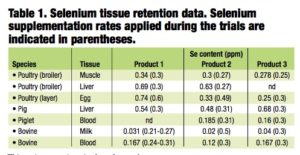
While the datasets are not directly comparable in terms of the trials used for the different products, the data do, nonetheless, clearly demonstrate that each of the individual products enhances the selenium content and retention in both tis- sue- and product-specific fashions.
In essence, all of the available data con- firm that selenized yeast products are distinct from each other. This distinct- ness is due to the differential deposition of selenium into the numerous peptides and proteins that are present within individual yeast fractions and indicates that retention and, thus, bioavailability of selenium from each of these products are different.
Conclusions
When comparing selenium yeast products, consideration needs to be given to the strain-specific manner in which selenium is deposited into individual protein and peptide-containing fractions.
These differences are ultimately responsible for the in ease with which digestion liberates selenoamino acids and the variation that exists in the bioavailabilities of individual products. Increasing the SeMet content does not necessarily increase the relative bioavailability of the selenium source.
Ultimately, not all selenium yeasts are created equally in terms of the availability of selenium-containing proteins and peptides.
